IMATINIB MESYLATE FOR AGGRAVATING HYPERPIGMENTATION
Dr Indu Ballani, Dermatologist, New Delhi, and Consultant Dermatologist, BLKMax Hospital, details how anti-cancer drug imatinib mesylate exacerbates hyperpigmentation.
A
44 –year-old female patient from New Delhi , married for 20 years, came to us with the complaints of extensive hyperpigmentation on her face for two years.
The patient was diagnosed with chronic myeloid leukemia for which she was prescribed imatinib mesylate tablet since four years. Two years into the treatment, she complained of hyperpigmented lesions, which first appeared on bilateral malar areas and progressed to involve forehead, nose and chin gradually over two months. She has been diagnosed with hypothyroidism four years ago and has been on thyroxine 75 mg tablets. She was not on any other medication.
Upon examination, there were multiple symmetrical brownish hyperpigmentation that were well-defined discrete to confluent macules present over bilateral malar, mandibular areas, forehead, chin and nose sparing central portion of forehead, periorbital areas and nasolabial folds. No other sites were affected. Nails, oral cavity, palms, soles and genital mucosa were unremarkable. The patient was treated previously with multiple topical medications like hydroquinone, tretinoin, steroids, kojic acid, glycolic, sunscreens and strict photoprotection was advised. Despite this, she did not report any improvement in her skin condition, and developed hypersensitivity and redness after using many of these preparations.
The patient reported slight improvement after two sittings of fractionated Q-switch laser. However, the hyperpigmentation worsened after a month. A PRP session was also performed which again resulted in the improvement of skin quality; however, there was little improvement in pigmentation.
Discussion
Imatinib is an anti-cancer drug with side-effects like photosensitivity, angular cheilitis, psoriasiform rash painful erosions, lichenoid eruptions and hypopigmentation. Hyperpigmentation is a rare side-effect that has been reported in a similar pattern in the patient one to two months after starting the drug. We observed photosensitivity, which was the initial symptom and hyperpigmentation, over two years. Although she is under strict sun protection, she has low tolerance to the procedures. Since the drug cannot be withdrawn, the patient has been counselled about continuing the usage of sunscreen and proper skin care ith planning of PRP sessions from time-to-time.

Fig 1
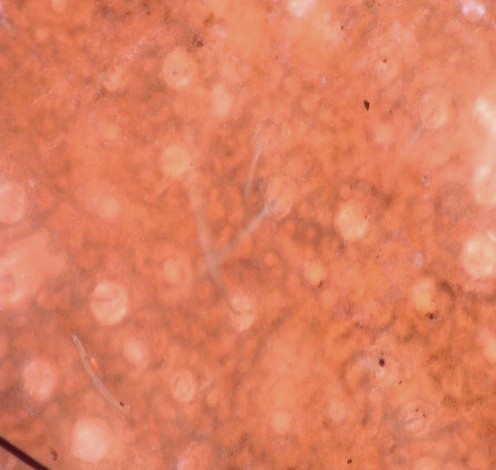
Fig 2
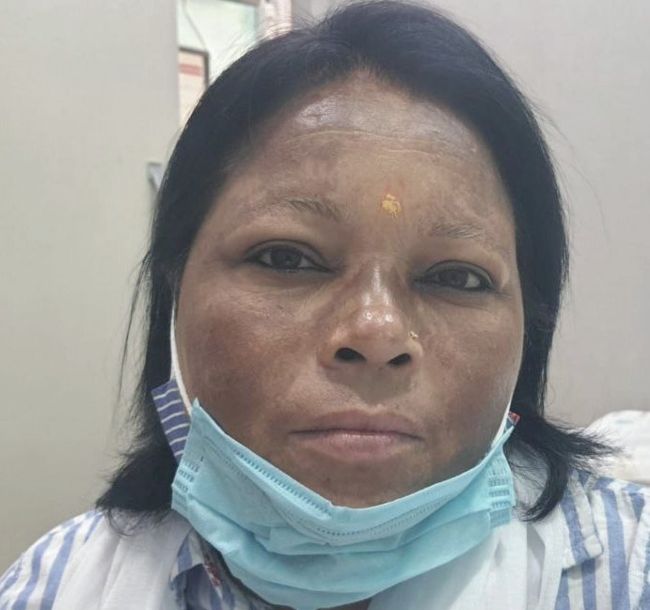
Fig 1 (Pre-PRP)
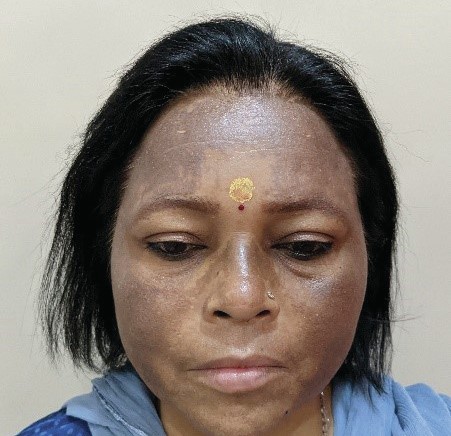
Fig
2 (Post-PRP)
On video dermatoscope, there were irregular dark brown pigment network in pseudo-reticular pattern, with few areas of hypopigmentation and perifollicular sparing of hyperpigmentation.