CLINICAL
Safely treating patients with HASHIMOTO’S DISEASE
Dr Lisa Dinley looks at the use of pegylated hyaluronic acid fillers
DR LISA DINLEY
Dr Lisa Dinley runs a private practice in Nottingham with her husband and loves the flexibility of treating patients in her own way, developing treatment protocols and carrying out clinical research. She is dedicated to improving evidence in aesthetic medicine and has published several articles in various journals. She is a member of the Elsvier advisory board and has peer reviewed many aesthetic and cosmetic surgery journals.
Cross-linked hyaluronic acid-based dermal fillers are used widely in aesthetic medicine and are available in varying degrees of densities and viscosities depending on the area and purpose of treatment. The most used cross-linking agent is butanediol diglycidyl ether (BDDE).1,4 Treatments with hyaluronic acid fillers are contraindicated in patients suffering from autoimmune diseases (which encompasses a large group of patients seeking treatments with hyaluronic acid fillers) due to an increased immune response. 2,3This can preclude many patients from undergoing rejuvenating and regenerating procedures, or if treated with BDDE fillers can risk unwanted side effects such as delayed hypersensitivity and granulomatous reactions.2,3 so, let’s discuss the use of hyaluronic acid-based fillers cross-linked with polyethylene glycol (PEG) and the benefits of this range of fillers.4,5,6 PEG fillers are extremely biocompatible, with a high safety profile, enabling them to even be used in patients with autoimmune disease.7 I will present a case of a patient with Hashimoto’s disease, successfully treated with pegylated fillers.
AIM
My patient is a 45-year-old female, suffering from Hashimoto’s disease. Hashimoto’s disease is an organ-specific autoimmune thyroid disorder affecting 10-12% of the population.
She wanted to look less tired and heavy through her jawline (fig 1-5) She had been turned away from several clinics previously because she had an autoimmune disease. She was extremely frustrated and getting very upset that she could not have any treatment.

Fig 1

Fig 2

Fig 3

Fig 4
Fig 5
After her initial consultation, it was noted that she had a heavy face, with a loss of volume and support to the midface and jawline. After discussing her non-surgical options with her, I felt confident that I could successfully treat her with the Neauvia filler range (Fig 6) that I use in my clinic.
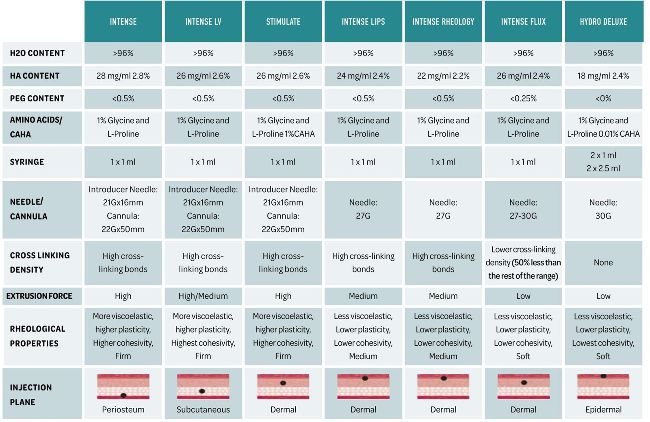
Fig 6 highlighting the full Neauvia range of fillers.
Neauvia fillers have a unique smart cross-linking technology (SXT) based on polyethene glycol (PEG). PEG is considered biologically inert, non-toxic, and nonimmunogenic.10 This confers a stealth technology to the filler, whereby the body accepts the filler as “self” and is not recognised as a foreign body.11,12
The SXT of PEG fillers confers a thermostability13 , allowing them to be used synergistically with energybased devices, enhancing treatment outcomes, in various protocols known as smart combination therapy. In this patient, we decided to employ the NLift protocol, a treatment targeted at the midface using Neauvia hydrodeluxe skin booster, fillers, the Zaffiro infra-red device and cosmeceuticals.14
There are many advantages to Pegylated fillers (Fig 7):
HASMIMOTO’S DISEASE
METHOD
The Nlift protocol is completed over two visits. The first visit is comprised of cleansing and toning the skin with Neauvia newborn skin and wake up skin, followed by administration of hydrodeluxe. Neauvia hydrodeluxe is a skin booster, containing 18mg/ml of hyaluronic acid, 0.1% of calcium hydroxyapatite and the amino acids glycine and L-proline. It provides deep hydration and collagen stimulation and is injected superficially. Visit one prepares the skin for the second part of the treatment. Asterile rigen mask containing hyaluronic acid and peptides is then applied to calm the skin followed by the application of a 30% vitamin C serum (C-shot) and ceramide shield. As part of the protocol, patients are given a kit containing fullsize C-shot and ceramide shield to support skin rejuvenation.
Aweek later, the patient returned, and Neauvia Intense filler was placed with a cannula over the lateral zygoma, pyriform fossa to create lift and at the pre-jowl sulcus. Intense filler has 28mg/ml of hyaluronic acid, with a high cohesivity and firmness to create structure. It has the highest lifting capacity of the whole range. Neauvia stimulate was placed with a cannula over the lateral cheek area and through the naso-labial fold and marionette. Stimulate has 26/ml of hyaluronic acid and 1% calcium hydroxyapatite, it is a unique scaffolding filler with a dual volumising and collagen-stimulating effect. Only 2ml of filler was used in total. Immediately following filler placement, the Zaffiro device was used. Hydroexfoliation followed by application of the infra-red energy. There were no issues immediately following treatment (fig 8-12)

Fig 8

Fig 9
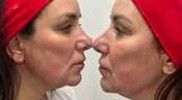
Fig 10 Fig 11

Fig 12
Fig 8, 9, 10, 11 and 12 immediately after treatment
RESULTS
The patient returned one month later to review the final result (Fig 13-17)

Fig 13

Fig 14

Fig 15

Fig 16

Fig 17
Fig 13, 14, 15, 16 and 17 one month after treatment
The patient was extremely satisfied with the outcome of the treatment and felt much more confident as a result. She was no longer self-conscious about her jawline. There were no issues with healing or integration of the filler and the result was a smooth, lifted appearance to the face, with improved skin quality and firmness. It is observed that there are less shadows cast on the face and more reflection of light. The naso-labial folds and marionette areas have improved, along with a reduction in the lid-cheek junction and smoother transition from the chin, pre-jowl sulcus and jawline overall. Although the chin appears longer and more balanced, no filler was placed in the chin.
CONCLUSION
As shown, Neauvia fillers are safe to use, even in patients with autoimmune disease. There are other benefits to Neauvia fillers too, including thermostability which has allowed me to improve my patient outcome in this case using the Nlift protocol.
I managed to achieve natural, rejuvenated results using only 2ml of filler in total. This is another benefit of the PEG cross linking, which confers unique viscoelastic properties to the range, with enhanced lifting and volumising effects, thereby less product is needed to achieve great results10 .
REFERENCES
1. Pierre, S.; Liew, S.; Bernardin, A. Basics of dermal filler rheology. Dermatol. Surg. 2015, 41, 120–126. [CrossRef] [PubMed]
2. Signorini, M.; Liew, S.; Sundaram, H.; De Boulle, K.L.; Goodman, G.J.; Monheit, G.; Wu, Y.; Trindade de Almeida, A.R.;Swift, A.; Vieria Brz, A. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers-Evidence-and Opinion-Based Review and Consensus Recommendations. Plast. Reconstr. Surg. 2016, 137, 961–971. [CrossRef]
3. Bailey, S.H.; Cohen, J.L.; Kenkel, J.M. Cosmetic medicine review article: Etiology, prevention, and treatment of dermal filler complications. Aesthetic Surg. J. 2011, 31, 110–121. [CrossRef] [PubMed]
4. Zerbinati, N.; Esposito, C.; Cipolla, G.; Calligaro, A.; Monticelli, D.; Martina, V.; Golubovic, M.; Binic, I.; Sigova, J.; Gallo, A.L.; et al. Chemical and mechanical characterization of hyaluronic acid hydrogel cross-linked with polyethylen glycol and its use in dermatology. Dermatol. Ther. 2020, 33, e13747. [CrossRef] [PubMed]
5. Rauso, R.; Nicoletti, G.F.; Bove, P.; Rauso, G.M.; Fragola, R.; Lo Giudice, G.; Zerbinati, N. Clinical Experience with PEGylated Hyaluronic Acid Fillers:A 3-year Retrospective Study. Open Access Maced. J. Med. Sci. 2021, 9, 1168–1173. [CrossRef]
6. Marino, F.; Cosentino, M.; Legnaro, M.; Luini, A.; Sigova, J.; Mocchi, R.; Lotti, T.; Zerbinati, N. Immune profile of hyaluronic acid hydrogel polyethylene glycol crosslinked: An in vitro evaluation in human polymorphonuclear leukocytes. Dermatol. Ther. 2020,33, e13388. [CrossRef]
7. Kubik, P.; Gallo, D.; Tanda, ML.; Jankau, J.; Rauso, R.; Gruszynski, W,; Pawlowska, A.; et al. Evaluation of the Safety of Neauvia Stimulate Injectable Product in Patients with Autoimmune Thyroid Diseases Based on Histopathological Examinations and Retrospective Analysis of Medical Records. Gels. 2023, 9, 440
8. Baharvand, P.; Hormozi, M.; Aaliehpour, A. Comparison of thyroid disease prevalence in patients with celiac disease and controls. Gastroenterol. Hepatol. Bed Bench. 2020, 13, 44–49.
9. Paiato, M. and ichert, S. Poly(ethylene glycol) Hydrogels with Adaptable Mechanical and Degradation Prroperties For Use in Biomedical Applications. Toxicology 2005, 214, 1-28
10. Zerbinati, N.; Esposito, C.; Cipolla, G.; Calligaro , A.;Monticelli ,D.; Martina,V.;et al.Chemical and mechanical characterization of hyaluronic acid hydrogel cross-linked with polyethylene glycol and its use in dermatology. DermatolTher. 2020;, 3
11.Marino,F.;Cosentino,M.;Legnaro ,M.; Luini ,A.; Sigova ,J.; Mocchi,R,.;Lotti,T,.;Zerbinati,N.Immune profile of hyaluronic acid hydrogel polyethylene glycol crosslinked:An in vitro evaluation in human polymorphonuclear leukocytes. DermatolTher. 2020 May;33
12. Webster R, Didier E, Harris P, Siegel N, StadlerJ, Tilbury L, Smith D. PEGylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug Metab Dispos. 2007 Jan;35(1):9-16. doi: 10.1124/dmd.106.012419. Epub 2006 Oct 4. PMID: 17020954.
13. Kubik, P.; Gruszczyński,W „Wpływ temperatury na różne wypełniacze na bazie kwasu hialuronowego”. Academy ofAesthetic and Anit-Aging Medicine 2019; 40-46.
14. Kubic, P.; Jankau, J.; Rauso, R.; Galadari H, Protasoni, M.; Gruszczynski, W.; Grzanka, D.; et al HA PEGylated Fillerr in Association with an Infrared Energy Device for the Treatment of Facial Skin Aging: 150 Day Follow-Up Date Report. Pharmaceuticals 2022, 15 1355